WHO Global Influenza Surveillance and Response System (GISRS) Surveillance and Vaccine Development
The WHO Global Influenza Surveillance and Response System (GISRS) monitors which influenza viruses are circulating in humans around the world throughout the year. GISRS comprises:
The major technical roles of GISRS are to:
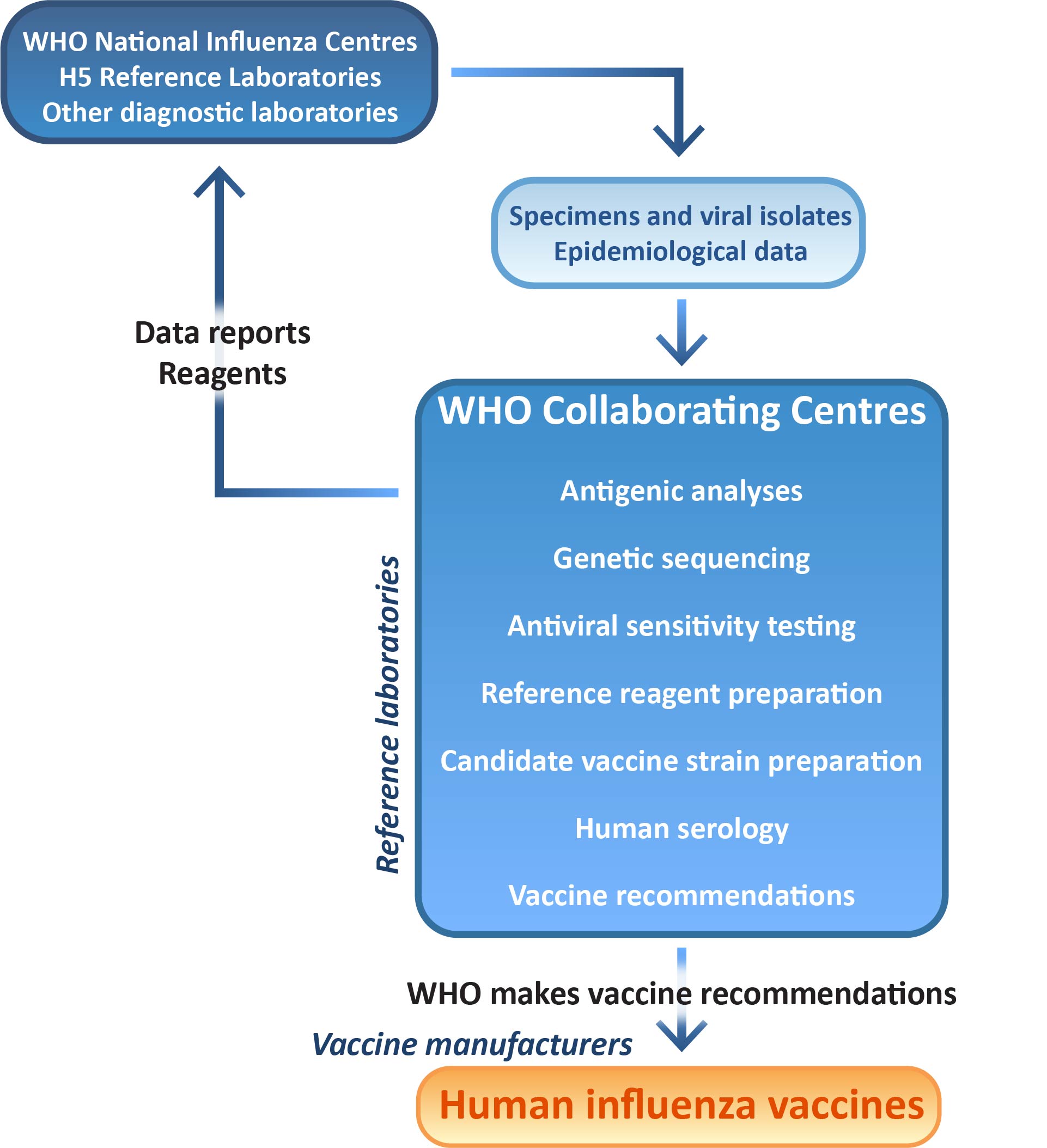
Influenza specimens and isolates obtained at WHO National Influenza Centres and Reference Laboratories are forwarded to a WHO Collaborating Centre, where they are analysed for antigenic (immune response), genetic and anti-viral drug sensitivity properties. Surveillance data about the circulating viruses are continually collected throughout the year and provide information about the predominant influenza viruses currently spreading and circulating in different parts of the world.The data posted by WHO National Influenza Centres is available on the WHO FLUnet website.
Haemagglutination inhibition (HI) assays measure the antigenic relationship between a test virus (Virus X) and a known reference virus (Virus A). Ferret antiserum raised against Virus A is tested for its ability to inhibit agglutination of red blood cells by Virus X, showing whether the haemagglutinin of the test virus has similar antibody-binding specificity to the reference virus, or is potentially a new strain.
Representative and antigenically unusual influenza strains are selected for full sequencing of their haemagglutinin and neuraminidase genes. New sequences are phylogenetically classified relative to known sequences, enabling genetic changes and evolution of circulating influenza strains to be tracked. Sequences are submitted to GISAID, an international database for sharing and collaborative use of influenza data.
Circulating viruses are tested for their sensitivity to influenza drugs that are both currently in use and in late-stage development. This monitoring enables WHO GISRS to identify the emergence of drug-resistant influenza viruses that could present future treatment challenges.
WHO Collaborating Centres work together with vaccine manufacturers to ensure the suitability of candidate strains for inclusion in seasonal vaccines. In accordance with regulatory requirements, WHO Collaborating Centres undertake primary isolation of selected viruses from clinical samples directly into eggs. Candidate vaccine viruses that are successfully isolated in eggs, post-infection ferret antisera raised against these viruses and other reference viruses are exchanged between WHO Collaborating Centres to enable direct comparison of strains isolated in the five centres.
Selected egg-isolated candidate vaccine strains are made available to the three laboratories that undertake virus reassortment for WHO — bioCSL (Australia), the National Institute for Biological Standards and Control (NIBSC, UK) and New York Medical College (NYMC, USA) — where they are reassorted with established egg-adapted strains to produce potential vaccine seed strains. The reassortant vaccine seed viruses are returned to the WHO Collaborating Centres, where they are analysed by HI assay and genetic sequencing to ensure that key antigenic and genetic properties of the vaccine virus have been retained.
Twice a year a WHO committee meets to consider the surveillance data on recently circulating viruses and candidate vaccine viruses, and recommend suitable strains to be included in the next seasonal influenza vaccines.
These recommendations are made 5-6 months ahead of vaccine release to allow time for production.
In each individual country, national authorities make the final decision on vaccine composition, usually in consideration of the WHO recommendation. In Australia, this decision is undertaken by the Australian Influenza Vaccine Committee (AIVC) at the Therapeutic Goods Administration (TGA).
Information for laboratories with influenza samples to send to a WHO Collaborating Centre is available here.
The WHO Pandemic Influenza Preparedness (PIP) Framework aims to improve the global preparedness in the event of an influenza pandemic through:
The PIP Framework was developed over the course of 4 years from 2007 and became effective in May 2011. Under the PIP Framework, countries share influenza viruses with pandemic potential with GISRS in a timely manner for analysis and development of candidate vaccine viruses.
Under the PIP Benefits Sharing System, manufacturers of influenza vaccines, diagnostics and pharmaceuticals that use GISRS make an annual monetary contribution (Partnership Contributions) to WHO. These contributions are used by WHO to fund capacity building projects in developing countries that require more support in influenza prevention and surveillance. Examples of such support may be training activities to strengthen laboratory surveillance capabilities, or strengthening systems to improve access to vaccines and medicines within those countries should a pandemic occur. In 2014, $15,048,215 was collected and a total of 26 countries received funds and technical support from Partnership Contributions.
The Centre participates in the PIP Framework through the receipt and analysis of potential pandemic viruses, as well as sharing of these viruses, data and expertise through approriate systems established through the Framework. The Centre also maintains its PC3 laboratories and regulatory approvals and ensures appropriate acknowledgement and research engagement of laboratories which originally provide the potential pandemic viruses.