HI Typing Assay
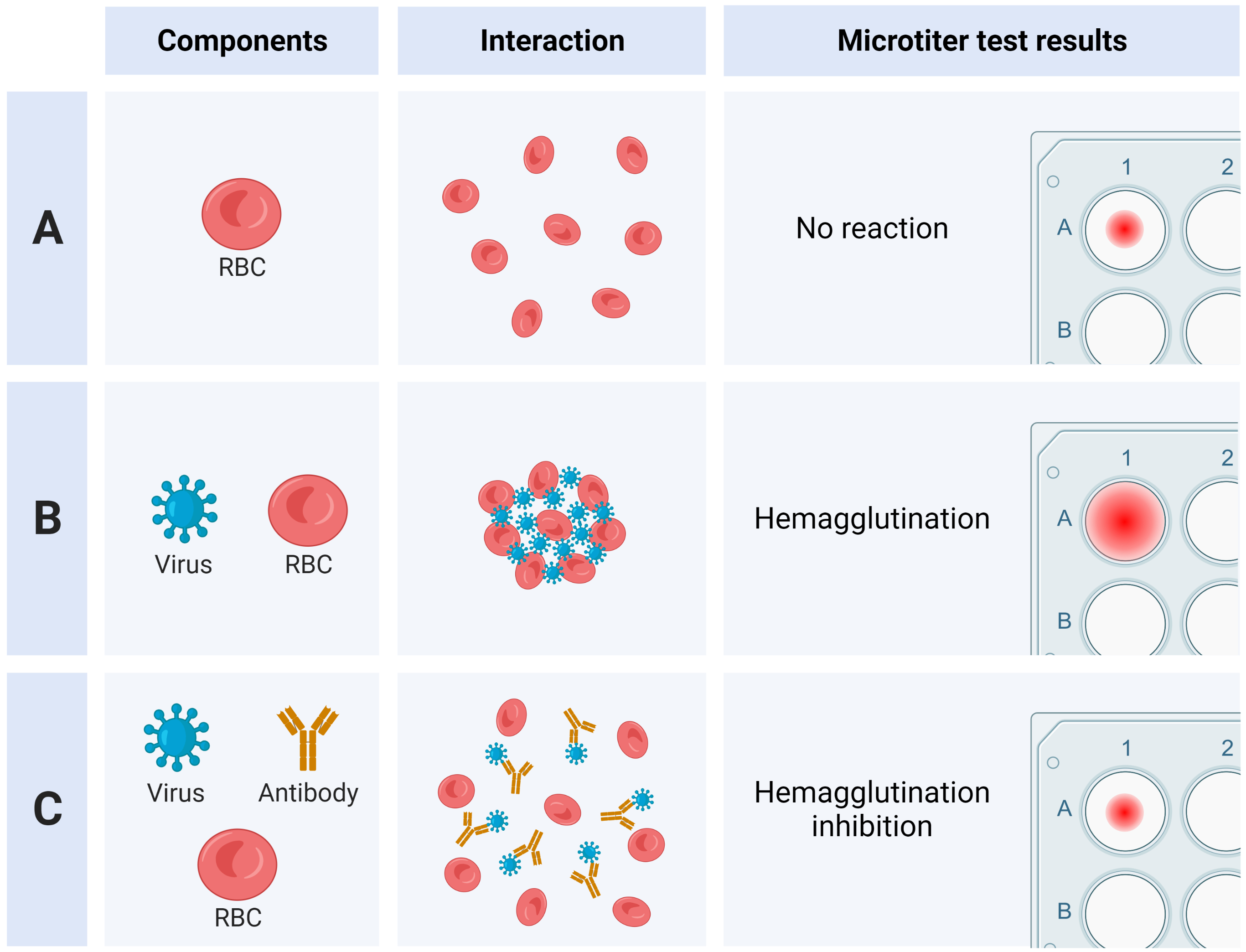
The Haemagglutination Inhibition (HI) Assay is used to identify and type new and existing virus isolates, using antisera prepared against known standard viruses.
As mentioned in the 'About influenza' section, influenza viruses are covered in surface proteins called haemagglutinin, which bind specifically to sialic acid-containing receptors. These receptors are found on the plasma membrane of certain cells, such as red blood cells (RBCs). When RBCs are mixed with influenza virus at the appropriate ratio, the virus binds these receptors (and therefore the RBCs), forming a lattice structure. This process is called haemagglutination.
Under normal conditions (without virus), when suspended RBCs are placed inside the well of an assay plate, the cells will slowly settle to the bottom of the well over time. In the presence of influenza virus, however, haemagglutination will alter how the RBCs settle.
Addition of antibodies/antisera (prepared against antigenic determinants like haemagglutinin) to this mixture interferes with, or inhibits the binding of viral haemagglutinin to these receptors on the RBC membrane.